Medications are substances given to prevent, treat, monitor or cure disease. They can be prescription or non-prescription and include complementary healthcare products (DoHaAC 2023).
Medications are crucial in maintaining health, preventing illness and treating health conditions - but when used inappropriately or incorrectly, they pose a serious risk of harm (Department of Communities Tasmania 2022).
For this reason, workers employed by National Disability Insurance Scheme (NDIS) providers must be able to competently manage medications for participants who require them.
What is Medication Management?
Medication management takes place at both an individual level and a system level. It may include:
- Selecting, ordering and supplying medications
- The way in which people take medications or are assisted to take them
- Recording and reviewing medications
- Storing and disposing of medications
- Supporting, monitoring and evaluating the use of medications.
(Department of Communities Tasmania 2022)

Types of Medications
Medications come in a variety of forms. Workers may need to administer:
- Tablets
- Capsules
- Wafers or melts
- Pastilles or lozenges
- Oral liquids
- Topical skin preparations
- Eye and ear drops
- Nasal drops or sprays
- Inhalants
- Transdermal patches.
(NDS 2016)
Management of Medication in the NDIS Practice Standards
Management of medication is a requirement of the NDIS Practice Standards, under Core Module 4: Provision of Supports Environment.
This Practice Standard aims to ensure that NDIS participants feel confident that their provider administers, stores and monitors the effects of the medications being administered, and that providers work to prevent errors and incidents (NDIS 2021).
Management of Medication Quality Indicators
- The provider’s records clearly state the medications and dosages required by each participant, including all information required to correctly identify the participant and safely administer the medication.
- Workers who are responsible for administering medications understand the potential effects and side effects of medications and know what steps to take if an incident involving a medication occurs.
- Medications are safely and securely stored, can be easily identified and differentiated, and can only be accessed by appropriately trained workers.
(NDIS 2021)
Medication Administration Records (MAR)
Medication Administration Records (MAR) monitor, review and reconcile a person’s medication information and administration (Department of Communities Tasmania 2022).
MARs are essential in reducing medication errors and incidents. They also:
- Support safe prescribing and administration
- Improve communication
- Ensure continuity in treatment between differing support settings.
(Department of Communities Tasmania 2022)
A participant’s MAR should be a current, accurate and reliable record of all their medications. This should include all prescription, non-prescription, complementary and alternative medications being used by the participant (Department of Communities Tasmania 2022).
Safe Medication Administration
In order to safely administer medicines, all workers must adhere to the following checking process known as the 13 Rights of Medication Administration:
- Right Person
- Right Medication
- Right Dose
- Right Route
- Right Time
- Right Effect
- Right Documentation
- Right Reason
- Right Expiration Date
- Right of the Person to Refuse
- Right Education
- Right Assessment
- Right Evaluation
(Ausmed 2019)
All medication administration should be documented (Department of Communities Tasmania 2022).
Medication Effects and Side-effects
Before administering medication, a worker should, as much as possible, understand:
- The reason why the participant is taking each medication
- How the medication should be administered
- Potential side effects and interactions with other medications.
(Department of Communities Tasmania 2022)
This information can be provided directly by the participant’s pharmacist or through written material, such as a consumer medicine information (CMI) leaflet.
CMI leaflets contain information on the safe and effective use of prescription and pharmacist-only medications. They provide information aimed at bringing about better health outcomes.
For more information on individual medications, search the NPS MedicineWise website.
Medication Incidents
A medication incident is ‘any event where the expected course of events in the administration of medications is not followed’ (Department of Communities Tasmania 2022).
Medication incidents include:
- Giving medication to the wrong person
- Giving the wrong medication
- Giving the wrong dose
- Giving medication at the wrong time
- Giving medication via the wrong route
- Missing a dose or giving an incomplete dose
- Spilling or dropping medication
- Losing a medication
- Medication being out-of-date
- The participant refusing medication or requesting not to be given medication
- A near miss.
(Department of Communities Tasmania 2022)
How to Respond to a Medication Incident
Note: The following is intended as a guide only. Always refer first to your organisation’s policies and procedures for the most appropriate course of action.
- Stay calm.
- Acknowledge that an error has been made. Try to identify the nature of the error and the cause of the error.
- Call an ambulance if the situation is an emergency.
- If appropriate, perform first aid as per DRSABCD.
- Call a colleague and/or your supervisor for advice and assistance.
- If the situation is not an emergency, continue to monitor the participant for changes in behaviour or wellbeing.
- If the participant is refusing to take medication, discuss this with them to find out why they are refusing. Explain why the medication is needed, wait for up to 30 minutes and then try offering the medication again.
- Call the participant’s prescribing healthcare professional, pharmacist or the Poisons Information Line on 13 11 26 and follow their instructions.
- Keep monitoring the participant for adverse reactions, or changes in behaviour or wellbeing.
- Record the error in the participant’s MAR and complete an incident report as per your organisation’s policies and procedures.
- Inform relevant staff about the error and provide information about the error during handover to other workers.
- Notify the participant’s family or substitute decision-maker, if appropriate.
- Clarify instructions for future medication administration and ensure future supply. For example, if the incident was related to a secure dosage administration aid (SDAA), have the aid repacked.
(Department of Communities Tasmania 2022)
Storage of Medications
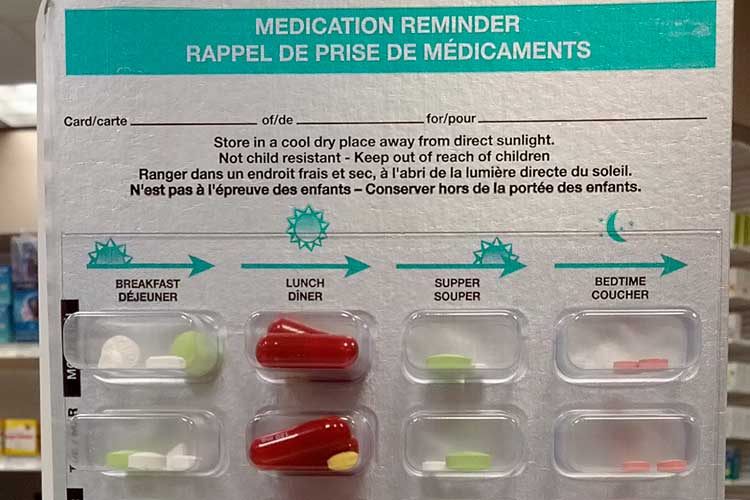
All medications must be safely and securely stored in a way that maintains the quality of the medication and protects those who live in, work in or visit the service environment.
Wherever possible, dispensed medications should be kept in their original packaging.
In some cases, a secure dosage administration aid (SDAA) might be deemed more appropriate for safely storing a medication.
An SDAA is a pharmacy-prepared aid that divides solid oral medications into sealed individual doses. The medications are arranged according to the dosing schedule throughout the day (Department of Communities Tasmania 2022).
Medications, including those in an SDAA, should be stored:
- In accordance with legislative requirements
- In their original packaging or a pharmacy-issued SDAA, and
- In a locked cupboard or room.
(Department of Communities Tasmania 2022)
The personnel in charge of the premises should clearly label and keep the keys to medication cabinets.
In most cases, medications should be stored in a cool, dark, dry and secure place. However, certain medications may require special conditions, such as refrigeration.
Medications should always be under the control of a specified worker. At the end of each shift, this worker should be responsible for checking the participant’s MAR to ensure that no medication administrations have been missed and that an accurate record has been kept (Department of Communities Tasmania 2022).
Identification and Differentiation of Medications
Workers must always check the medication name on the packaging before beginning to prepare the medication for administration. The medication name on the packaging must be exactly the same as the medication name on the participant’s MAR and should be easily identified and differentiated by the active ingredient and medication strength.
Note that medications can have several different names:
- The active ingredient name is a precise description in chemical terms of what the medication is
- Brand names are the names given to the medication by the pharmaceutical companies that manufacture it
- The original brand name is the first patented brand of the medication
- Generic brand names are other versions of the medication developed by other companies.
(NPS MedicineWise 2021)
For example:
| Active ingredient name: | Sertraline |
| Original brand name: | Zoloft |
| Generic brand names: | Eleva, Sertra, APO-sertraline, Sertraline Sandoz, Setrona |
(PBS 2016)
For this reason, it is advised to use active ingredient names rather than brand names.
Medications might come in a box, bottle or another kind of packaging. They could also be packed in a pharmacy-issued SDAA, i.e. a blister pack or sachet. Medications that are not in either their original packaging, suitable packaging as supplied by a pharmacist or in a pharmacy-prepared SDAA should not be administered as they cannot be easily identified or differentiated. This means the potential for error is high and it is not possible to comply with the 13 rights of medication administration.

Appropriate Training of Workers
Disability service providers should ensure the workers they employ are competent to provide appropriate and safe medication management support to people living with disabilities. Workers should not administer medications until they have completed training and are deemed competent by an appropriate training organisation.
Test Your Knowledge
Question 1 of 3
Why is it advised to use the active ingredient name of a medication rather than a brand name?
Topics
Further your knowledge
References
- Ausmed 2019, ‘13 Rights of Medication Administration’, Ausmed, 11 September, viewed 28 August 2024, https://www.ausmed.com.au/cpd/explainers/13-rights-of-medication-administration
- Department of Communities Tasmania 2022, Disability Services Medication Management Framework, Tasmanian Government, viewed 28 August 2024, https://www.dpac.tas.gov.au/divisions/cpp/community-and-disability-services/publications/policies,_procedures_and_guidelines/medication_management_framework
- Department of Health and Aged Care 2023, Medication Management in the Community: Guiding Principles, Australian Government, viewed 28 August 2024, https://www.health.gov.au/resources/publications/guiding-principles-for-medication-management-in-the-community?language=en
- National Disability Services 2016, Get Ready to Assist Clients With Medication, NDS, viewed 28 August 2024, https://nds.org.au/item/get-ready-to-assist-clients-with-medication
- NDIS Quality and Safeguards Commission 2021, NDIS Practice Standards: NDIS Practice Standards and Quality Indicators, Australian Government, viewed 28 August 2024, https://www.ndiscommission.gov.au/sites/default/files/documents/2019-12/ndis-practice-standards-and-quality-indicators.pdf
- NPS MedicineWise 2021, Medicines and Brand Names, Explained, Australian Commission on Safety and Quality in Health Care, viewed 28 August 2024, https://www.nps.org.au/consumers/medicines-and-brand-names-explained#why-does-the-same-medicine-have-different-brand-names?
- Pharmaceutical Benefits Scheme 2016, Sertraline, Australian Government, viewed 28 August 2024, https://www.pbs.gov.au/medicine/item/2236Q-8836C
 New
New 

