After reading and digesting this article, the practising healthcare professional should be able to discuss the meaning of the terms pharmacokinetics and pharmacodynamics, and:
- Compare and contrast pharmacokinetics and pharmacodynamics
- Discuss the importance of creatinine clearance
- Discuss the importance of half-life of a medicine
- Discuss the importance of the P450 metabolic pathway.
What is Pharmacology?
Medicines aim to prevent, cure or control various disease states. To achieve this goal, adequate concentrations of the medicine must be delivered to the target tissues so that therapeutic, yet non-toxic levels, are obtained.
Pharmacological and toxicological actions of medicines are primarily related to their plasma concentrations. Consequently, healthcare professionals who work with medicines must recognise the onset speed of medicine action as well as the intensity and duration of its effect. In turn, these are controlled by the following four fundamental pathways of drug movement and modification in the body:
- Absorption
- Distribution
- Metabolism
- Excretion, as well as onset and duration of action.
Pharmacokinetics v Pharmacodynamics
Pharmacokinetics influences the decided route of administration for a specific medication, the amount and frequency of each dose, and its dosing intervals.
Pharmacodynamics, on the other hand, is the study of how a medicine acts on a living organism . This includes the pharmacological response and its duration and magnitude observed, relative to the medicine's concentration at an active site in the organism, i.e. the study of a medicine's effect and the mechanisms of action.
What Medicines Do to the Body
Clinical pharmacokinetics is the application of pharmacokinetic and pharmacodynamic principles to the safe and effective therapeutic management of an individual patient.
Half-Life Formula
The pharmacokinetic term half-life (t1/2) refers to the time taken for half the initial dose of medicine administered to be eliminated from the body. That is:
For example, a medicated dose with a half-life of 12 hours would have 25% of the medication left in the body after 24 hours.
Many medications are classified in terms of their half-lives. For example, the benzodiazepines are classified in terms of:
- Ultra-short acting (t1/2 < 6 hours): midazolam, triazolam
- Short-acting (t1/2 6-12 hours): oxazepam, temazepam
- Medium-acting (t1/2 12-24 hours): alprazolam, bromazepam, lorazepam
- Long-acting (t1/2 > 24 hours): clobazepam, clonazepam, diazepam, flunitrazepam, nitrazepam.
Absorption, Distribution, Metabolism and Excretion
Below is a schematic representation of the absorption, distribution, metabolism and excretion of medicines:
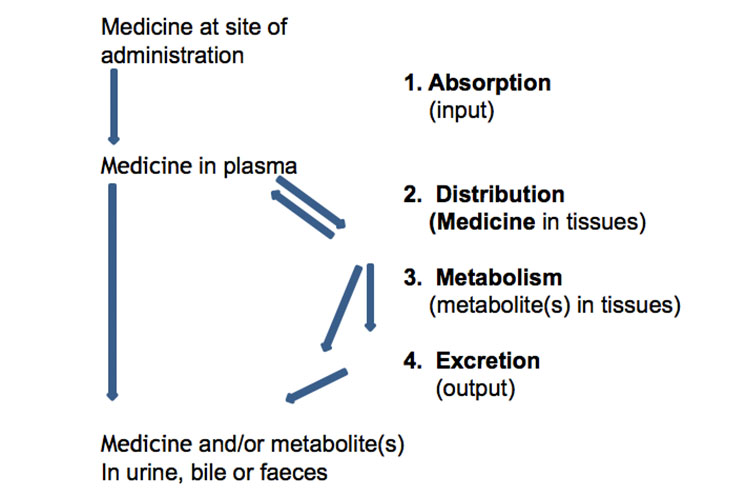
Alternatively, the following graph represents a medicine's absorption, distribution, metabolism and excretion, along with some pharmacokinetic terms, after a single oral immediate-release dose:
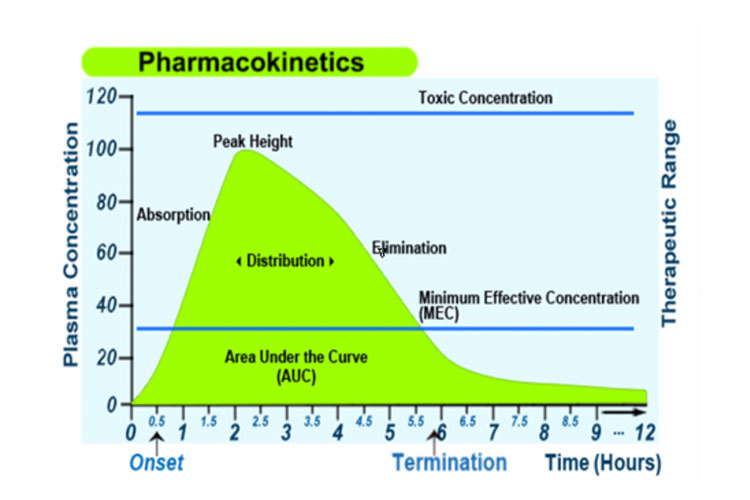
Absorption
Absorption from the site of administration permits entry of the therapeutic agent (either directly or indirectly) into plasma.
Medicine-related factors include ionisation state, molecular weight, solubility and formulation. Small, nonionised, lipid-soluble medicines permeate plasma membranes most readily.
Distribution
Once absorbed, the medicine may then reversibly leave the bloodstream and distribute into the interstitial and intracellular fluids.
A medicine's permeability is defined by the blood-brain barrier, blood-testes barrier and blood-placenta barrier.
Most medicines are protein-bound to some extent; only an unbound medicine is free to carry out its pharmacological action(s).
Depot Storage refers to lipophilic medicines that store in fat, calcium-binding drugs, etc.
With ageing, there is a reduction in lean body mass and total body water content, and an increase in total body fat. This can result in changes in the volume of distribution (Vd) for some medicines, causing unpredictable effects, particularly in frail older adults.
The volume of distribution is the extent to which a medicine distributes out of the bloodstream and into the tissues of the body (i.e. the site of drug receptors). A decrease in Vd will result in higher plasma concentrations for hydrophilic medicines such as gentamicin, digoxin and lithium. A higher proportion of body fat will increase Vd for lipophilic medicines such as diazepam, causing an increase in plasma half-life.
Metabolism
Before being excreted, the medicine is metabolised by the liver, kidney or other sites. This is the process of making the drug more polar (more water-soluble), which may lead to medicine inactivation and excretion.
Metabolites may be more or less (prodrug) active than the parent medicine. The liver is the major source of these enzymes (P450 enzymes), though they may be present in the gastrointestinal tract, heart, lungs, brain and kidneys.
1. Phase I Reactions (nonsynthetic)
Phase I reactions (nonsynthetic) involve minor structural modifications of the parent structure via oxidation, reduction or hydrolysis to produce smaller, more water-soluble metabolites. These are predominantly handled by the Cytochrome P450 enzymes.
Phase I reactions frequently provide a 'handle' for further modifications by subsequent Phase II reactions.
2. Phase II Reactions (synthetic)
Phase II reactions (synthetic) involve the coupling of a water-soluble endogenous molecule such as glucuronic acid, sulphate or glutathione to a chemical (parent compound and/or Phase I metabolite) to facilitate excretion.
The most common causes of medicine-to-medicine interactions are pharmacokinetics, particularly metabolic ones. These are known as cytochrome P450 interactions.
A large number of clinically important interactions arise from inhibition or induction of substrates (medicines that are significantly metabolised by the given enzymes).
- Inhibitors are compounds that are generally capable of inhibiting the metabolism of the various substrates. As a result, administration of the inhibitor may lead to an increased plasma concentration of the substrate. For example, ciprofloxacin inhibits the CYP3A4 enzyme that metabolises clozapine, which may lead to a toxicity of clozapine.
- Inducers of the specified P450 have the capacity to increase the activity of the designated enzyme and, therefore, reduce the plasma concentrations of the listed substrates. For example, carbamazepine induces the metabolism of cyclosporin via the CYP3A4 enzyme, leading to a reduction in plasma levels of cyclosporin and, hence, loss of efficacy.

Excretion
Medicines and their metabolites are removed from the body in urine, bile and/or faeces. Some cases first need to be metabolised to more water-soluble moieties (examples include amiodarone, amitriptyline, amlodipine, amphotericin B, aripiprazole, aspirin, atomoxetine, atorvastatin, azithromycin, felodipine etc.)
Others are excreted unchanged or relatively unchanged (penicillins, cephalosporins, aminoglycosides, ganciclovir, milrinone, oseltamivir, risedronate, varenicline etc.).
The main processes involved in excretion are glomerular filtration, tubular secretion and tubular reabsorption.
Tubular excretion and reabsorption may also be influenced by medications that either make the urine more acidic (ammonium chloride, large doses of vitamin C) or more alkaline (urinary alkalisers such as Citralite®, Citravescent®, Uracol®, Ural® , which all raise the urinary pH to about pH 9).
Creatinine and Creatinine Clearance
Creatinine (Normal range: Females 50-110 micromol /L; Males 60-120 micromol /L) is the metabolic product of muscle metabolism. Its level is a reflection of both muscle mass and kidney function, as creatinine is predominantly eliminated by glomerular filtration through the kidney.
Low levels may indicate protein starvation, liver disease or pregnancy, whereas high levels are seen in kidney failure, muscle degeneration and the effects of some medicines that block renal secretion (e.g. cimetidine and trimethoprim).
Creatinine clearance (CrCl)
Creatinine clearance (CrCl) is an estimate of the glomerular filtration rate (GFR), which is a direct measure of renal function. The CrCl can be calculated by means of the Cockroft-Gault Equation:
(For females, multiply by 0.85 to account for the reduced muscle to ideal body weight ratio in comparison to males.)
- Ideal Body Weight for Males = 50 kg + 0.9 kg/each cm above 152 cm.
- Ideal Body weight for Females = 45.5 kg + 0.9 kg/each cm above 152 cm.
(Many monographs on medicines will indicate dose and dose frequency of a medicine according to the Creatinine Clearance.)
eGFR
eGFR is used by pathology labs as an estimated indicator of renal function. (eGFR has limitations, and for medicine dosing when renal function is < 60 mL/min, the Cockcroft-Gault equation is preferable.)
Bioavailability
Bioavailability is a subcategory of absorption and is the fraction of an administered dose of unchanged medicine that reaches the systemic circulation. By definition, when a medication is administered intravenously, its (absolute) bioavailability is 100%.
Efficacy of a drug is a function of the rate and extent of absorption. Relative bioavailability is derived as a relationship (%) between the bioavailability from an oral dose-form (or other non-IV dose) relative to an IV dose.
Test Your Knowledge
Question 1 of 1
Which one of the following statements is not correct?
Topics
References
- Bryant, B & Knights, K 2015, Pharmacology for Health Professionals, 4th edn, Mosby Elsevier, Sydney, Australia
- eMIMs 2016, CMPMedica, https://www.mimsonline.com.au/Login/Login.aspx?ReturnUrl=%2fdefault.aspx
- Harris, P, Nagy, S & Vardaxis, N 2014, Mosby’s Dictionary of Medicine, Nursing and Health Professions, 3rd edn, Elsevier, Australia.
- Neal, M J 2015, Medical Pharmacology at a Glance, 8th edn, Blackwell Science, Oxford.
- Rossi, S 2016, Australian Medicines Handbook (AMH), https://amhonline.amh.net.au/auth
 New
New 
