This article is part of our Training Requirement Series, where we provide comprehensive guides to meet the actual training requirements that are often needed/requested of learning and development departments within Australia's healthcare organisations. This series includes both general requirements, such as adverse drug reactions, but also focuses on the specific requirements stemming from the NDIS, Aged Care and NSQHS Quality Standards.
What are Adverse Drug Reactions?
An Adverse Drug Reaction (ADR) is an unintended and harmful effect resulting from the administration of medication (Aronson & Ferner, 2005). ADRs can manifest in numerous ways, such as allergic reactions, side effects, or interactions with other medications. They can be classified into two main categories:
- Type A: Augmented reactions, which are dose-dependent and predictable.
- Type B: Bizarre reactions, which are dose-independent and unpredictable.
The risk factors contributing to ADRs may include the age of the patient, polypharmacy, and comorbid conditions.
How Prevalent are ADRs in Australia?
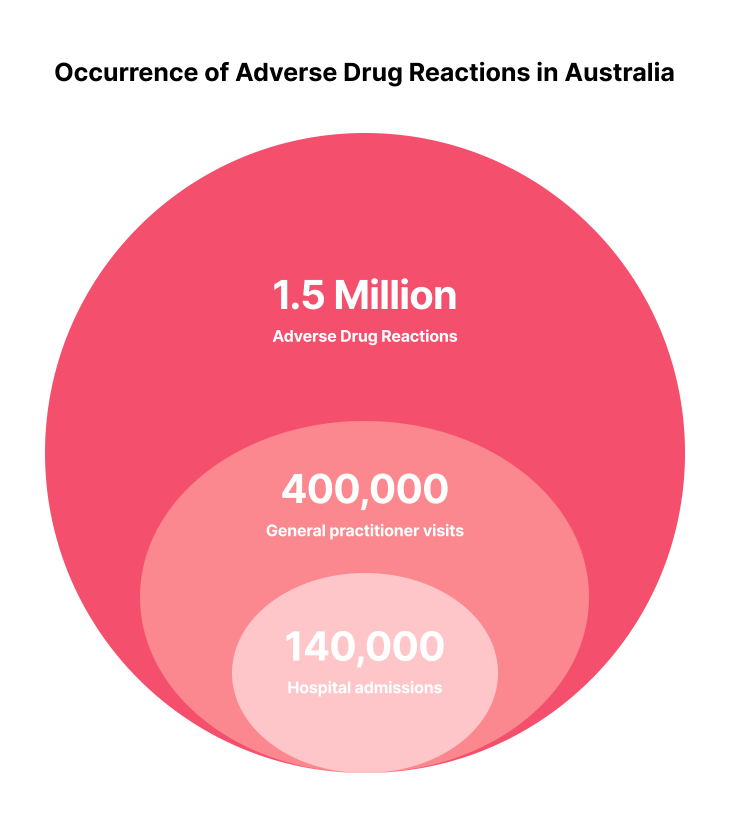
According to the Australian Commission on Safety and Quality in Health Care (2019), approximately 1.5 million Australians suffer an adverse event from medicines each year, resulting in at least 400,000 visits to general practitioners and 140,000 hospital admissions. This highlights the significance of proper training and management strategies in healthcare settings. Not only do these ADRs significantly impact those suffering from the events, but they also drive financial costs to the healthcare industry.
This highlights the significance of proper training and management strategies in healthcare settings.
What is the "Adverse Drug Reactions" Training Requirement?
To address this prevalent issue, it is vital to provide training in accordance with the National Safety and Quality Health Service (NSQHS) Standards, Aged Care Quality Standards, and Strengthened Aged Care Quality Standards. This training should encompass the identification, prevention, and management of ADRs and should be ongoing to keep healthcare professionals up-to-date with the latest best practices.
Relevant Standards
Action 4.07: Collecting adverse drug reaction history:
The healthcare organisation has a patient documentation process to collect a patient's history of medication allergies, including a record of prior adverse drug reactions.
Action 4.08: Adverse drug reaction documentation processes:
Processes for documenting adverse drug reactions that patients experience under the provision of care in the healthcare record are in place and included in organisation incident reporting systems.
Action 4.09: Therapeutic Goods Administration reporting:
Processes are in place to report adverse drug reactions to the Therapeutic Goods Administration (in accordance with its requirements)
National Safety and Quality Health Service (NSQHS) Standards
Requirement 3.3 (b): High-impact or high-prevalence risks:
Effective management of high-impact or high-prevalence risks associated with the care of each consumer
Action 5.3.3 (e): Medication review processes:
Medication reviews are conducted when there is an adverse event that is potentially related to medicines
Action 5.3.4: Medication documentation:
The providers must:
- Document existing or known side effects or allergies at the commencement of care
- Monitor and update documentation when new side effects or allergies occur
Action 5.3.6: Therapeutic Goods Administration reporting:
The provider reports adverse medicine and vaccine events to the Therapeutic Goods Administration.
Strengthened Quality Standards framework analysis - Aged Care Quality Standards
Failure to comply with the requirements could lead to an organisation being penalised or reprimanded.
Related Training Requirements Guides
The following Training Requirement guides can be used to support and facilitate the adverse drug reactions training requirement:
Skills Required by Staff for Adverse Drug Reaction Prevention and Management
Equipping staff with the right skills is critical to minimise the impact of ADRs on patients and the duration of hospital stays. Here are the skills in detail:
| Skill | Key Elements |
|---|---|
| Identification | Staff should be skilled in recognising the early signs and symptoms of ADRs. This includes changes in vital signs, patient complaints, and unusual laboratory findings. |
| Prevention | Healthcare staff must understand proper medication administration techniques, including dosage calculations, timing, and routes of administration. |
| Management | Skills in immediate intervention are essential. This involves activating emergency protocols and administering antidotes or other necessary treatments. |
| Communication | Staff must effectively communicate with team members and superiors to ensure comprehensive care. |
| Documentation | Proper recording of all medication administered and any observed ADRs is vital for both patient safety and legal compliance. |
How to Assess Staff Competency on Adverse Drug Reactions
Effective assessment of staff competency is multi-faceted:
- Written Exams: To assess theoretical understanding.
- Practical Demonstrations: To validate skills in medication administration and ADR identification.
- Competency Checklists: Ongoing competency checks during real-world scenarios can offer valuable insights into a staff member's abilities.
- Peer Reviews: Colleagues can offer valuable perspectives on a staff member's competencies.
By regularly assessing staff competence, you can identify areas for improvement and tailor training accordingly.
Strategies to Support Healthcare Staff Reinforce Adverse Drug Reaction Knowledge
Training should not end after an initial induction; it should be a continuous process. Training for ADR prevention and management can be performed (and not limited to) in the following ways:
- Mentorship: Experienced staff can provide hands-on training and guidance.
- Simulation Training: Simulated environments allow staff to hone their skills without endangering patients.
- Workshops: Targeted workshops can focus on specific skills or scenarios, providing intensive training opportunities.
- Continuous Feedback: Regular performance evaluations help identify areas for improvement.
Sample Training Plan for the Adverse Drug Reactions Training Requirement
The ability of staff to manage and prevent ADRs can be reinforced through an effective training program upon identifying the areas of lacking skills.
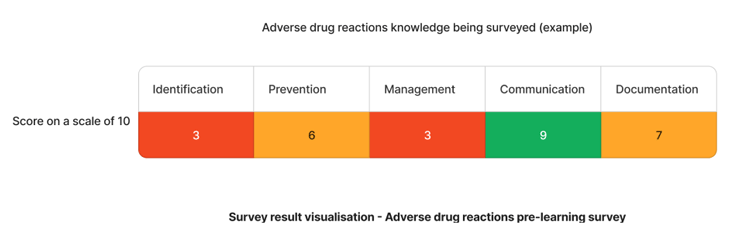
Using the above needs assessment survey as an example - The adverse drug reaction skills that require the most attention are identification and management skills. We can target learning initiatives to fill these gaps to enhance staff competency.
Need an LMS that can support adverse drug reaction training?
Contact Ausmed today and see how we can support with your adverse drug reactions training requirements!
Staff Competency Assessment for Adverse Drug Reactions - Example
To assess your healthcare staff competence in Adverse Drug Reactions, consider asking these survey questions:
Staff Survey - Adverse Drug Reactions Competency
-
Can you list three common signs of an adverse drug reaction?
- [Sign 1]
- [Sign 2]
- [Sign 3]
-
How would you respond to a suspected ADR?
- [Answer here]
-
Describe an escalation procedure in case of a severe ADR.
- [Answer here]
-
How would you document an ADR?
- [Answer here]
Conclusion
Addressing the significant issue of ADRs in Australia requires a comprehensive, multi-faceted training program. Ensuring that healthcare professionals are adequately trained and continually assessed can substantially mitigate the risks and improve patient outcomes.
References
- Aronson, J. K., & Ferner, R. E. (2005). Clarification of terminology in medication errors. Drug Safety, 28(10), 851-870.
- Australian Commission on Safety and Quality in Health, 2023. 'NSQHS Medication Safety Standard'
- Australian Commission on Safety and Quality in Health, 2023. 'NSQHS Action 4.07'
- Australian Commission on Safety and Quality in Health, 2023. 'NSQHS Action 4.08'
- Australian Commission on Safety and Quality in Health, 2023. 'NSQHS Action 4.09'
- Aged Care Quality and Safety Commission, 2023. Aged Care Quality Standards, ' Standard 3. Personal care and clinical care - Requirement 3.3.(b)'
- Aged Care Quality and Safety Commission, 2023. 'Stronger Standards, Better Aged Care Program - Action 5.3.3 (e)'
- Aged Care Quality and Safety Commission, 2023. 'Stronger Standards, Better Aged Care Program - Action 5.3.4'
- Aged Care Quality and Safety Commission, 2023. 'Stronger Standards, Better Aged Care Program - Action 5.3.6'


